Battery knowledge
On this page, we have summarized everything you need to know about batteries. First you will find a lexicon with all the essential definitions of terms that we and our customers often encounter. Our lexicon does not claim to be exhaustive. We try to explain important terms from our field of activity. We welcome further ideas and suggestions. You will then find more detailed information about batteries in the form of a brief summary of the history of the battery.
A
Accumulator / Rechargeable battery
An accumulator or rechargeable battery is a rechargeable reservoir of electrical energy that works electro-chemically. It stores electrical energy by converting it into chemical energy, and vice versa.
Acid density
The acid density contains information about the state of charge of a lead-acid battery. It is measured with an acid siphon. A fully charged battery has an acid density of 1.28 kg/l and a discharged battery ca. 1.1 kg/l.
Acid stratification
When charging a battery, the aim is to achieve a high acid density. The acid sinks to the lower part of the battery container. After repeated charges, variable densities form in the battery acid – higher densities below and lower densities above. It's important not to maintain the battery in this state for any length of time because there is a risk of it being destroyed. The major battery brands offer automatic acid circulation devices that ensure that the acid density remains constant.
AGM
Safe, powerful and durable!
Powerful modern batteries are based on AGM technology (Absorbent Glass Mat). Special micro glass-fiber mats lie in between the battery’s lead plates and absorb all the battery acid. The glass fiber mats is designed to absorb and hold all the acid, but without fully saturating the mat. The sealed system is equipped with a VRLA (Valve Regulated Lead Acid), a pressure-relief valve that safely disposes of any gases.
The technology offers many advantages:
The battery remains dry and leak-proof. In the case of frost, the expanding liquid can’t cause any damage. Because of the micro glass-fiber mats, there is scarcely any plate movement anymore, which means that the battery is unaffected by jolts and vibrations. Thus, AGM batteries can even be assembled on their sides and operated in any environment. In addition, because the acid is completely trapped in the mat, maintenance considerations such as water refills and monitoring the electrolyte are unnecessary! The way they are constructed means that AGM batteries have an extremely low internal resistance. Reactions between the acid and plate material are faster, and so, for example, in a challenging situation like charging in the extreme cold, more energy can be transferred! Charging AGM batteries is designed to be as simple as possible. A conventional car battery charger will work, you don’t need specialist chargers or adapters!
Alkaline
The galvanic alkaline battery is one of the most important electro-chemical energy storage systems. It has supplanted the zinc-carbon for many uses because of its higher capacity, better load capacity and longer storage capacity. Alkaline batteries are primary batteries, so they are non-rechargeable.
The main alkaline battery types are cylindrical round-cell (e.g. LR6 = Alkaline AA or Mignon) and button-cell.
Ampère [A]
The unit of electrical current, shortened to: A.
Ampere hours [Ah]
Ampere hours are the unit of electrical charge. It is the product of the current (measured in Amperes) and time (measured in hours). Ampere hours are typically the unit that the quantity of electric charge (capacity) of a battery or cell is given in. It is shortened to Ah.
Anode
The anode is the counterpart to the cathode and is where oxidation in a liquid solution occurs and electrons are released. During this process, anions (negatively charged electrons) are discharged. Both electrodes can function as the anode, depending on the direction of the electrical current in the secondary cells. During discharge, the negative electrode becomes the anode.
B
Back-up battery
A replacement/auxiliary power supply that is switched constantly at float voltage.
Battery
The battery is an electro-chemical energy reservoir. It is usually made up of a combination of electro-chemical cells – the so-called galvanic elements. The cells contain two electrodes that are kept separated by an ion-conducting fluid or solid electrolyte. Different battery types will have different voltages and energy densities depending on which chemical system they use. The material that the electrodes are made from defines how high the nominal voltage is, whereas the amount of energy that can be stored depends on the composition and quantity of the battery material. When the battery is discharged, chemically stored energy is converted to electrical energy through an electro-chemical reaction. The main parameters here are: hold-up time, apparent power, cos.Phi of the power user, inverter efficiency rate, link voltage, discharge voltage and charging voltage.
Battery types:
- Sealed battery
- Vented battery
- Open battery
Battery capacity
A battery’s nominal capacity is the capacity that it has when discharging over a specific discharge period (nominal discharge time tN), at a nominal temperature, nominal density and nominal electrolyte condition without it falling below the cutoff voltage (COV). Capacity values typically apply to a 10- or 20-hour discharge period for lead batteries and a 5 hour discharge for nickel-cadmium batteries. When used in UPS installations, short discharge times mean the batteries' working capacity is much smaller than the nominal capacity. Therefore, to get an accurate calculation you need tables or curves showing how discharging performance relates to discharge time. In addition, it is worth noting that some batteries reach their full nominal capacity after several charging cycles – i.e. indicating a smaller capacity than expected at the beginning may not be a sign of a defective battery.
Battery interface cabinet
A unit housing battery connection in a cabinet to accommodate the DC battery switch and cabling.
Battery pack
A battery pack refers to a package of inter-connected rechargeable battery cells that are housed in a container for practical reasons – often to shield it from environmental factors. Battery packs are designed to be replaceable/removable and have removable electrical contacts such as plug connectors.
To ensure that voltage, capacity and load capacity are as uniform as possible, the cells should all be the same type and made by the same manufacturer. Inside a battery pack, blocks containing several cells can be connected in parallel, and likewise, many blocks in a row.
Battery room
For particularly large battery installations, a dedicated room is often constructed in the building. The specifications for equipping a battery room are set out in DIN VDE 510 Part 2. These cover aspects such as ventilation and extraction, and adequate spacing for combustible and spark-generating elements.
C
Capacity
Describes the available quantity of storage or power of a battery or cell (measured in Ampere hours). Battery temperature and discharge current are integral to calculating capacity, therefore that data must necessarily be provided. (e.g. cold starting capacity measured in seconds, plus cold cranking current and temperature -18°C)
Capacity (Nominal)
This describes capacity under normal conditions and depends on battery type (e.g. 20 hour capacity in Ah, reserve capacity during discharge: 25 A in min.)
Capacity (Residual)
The residual capacity describes the useable capacity of a battery with an unspecified state of charge when being discharged at a nominal current (e.g. after a prolonged period of downtime).
Cathode
Describes the negative electrode where the process of reduction happens in a fluid solution – i.e. the cations (positively charged ions) are deposited (gaining an electron). In secondary (rechargeable) cells, either electrode can become the cathode depending on the direction of the current. The cathode is the positive electrode during discharge.
Cell
The smallest unit of a battery consisting of positive and negative electrodes, a separator and the electrolyte. It is the primary building block of a battery. The cell stores electrical energy, with the size of the cell being defined by its capacity.
Charge efficiency
The charge efficiency describes the ratio between used capacity and chargeable capacity, which is 0.85 in the case of lead batteries.
Charge factor
The charge factor describes the ratio between discharged battery capacity and capacity needed to recharge the battery. Because of their energy efficiencies, lead batteries need to be charged ca. 20% more and NiCd batteries ca. 40%.
Charging curves
The following curves are used to charge batteries:
- I curve
- IU curve
- U curve
- W curve
- Wa curve
Which charging curve should be used depends on the battery type. You can find out which one to use by referring to the manufacturer’s user manual.
I curve
This means charging with a constant current. Here charging voltage is not controlled, which can lead to the battery temperature rising, the electrolyte boiling and water loss. It may also cause a chemical reaction where hydrogen is released, raising the risk of an explosion. This charging method is only suitable for low charging loads of a couple of milliamps.
IU curve
An empty battery is charged with a constant current (I curve). The charge voltage is set at the float voltage. As the charge capacity of the battery increases, the charging current gradually decreases, and the charge voltage rises until it reaches the intended float voltage (2.26V/cell – 2.29V/cell, depending on the type of lead battery). At this point the charger switches to the U curve.
U curve
Charging at a constant voltage. If a high voltage charge of 2.4V/cell is reached, the current is automatically reduced to prevent the battery temperature from rising too much (An overly high temperature can destroy the battery).
W curve
Charging follows a resistance curve (W). As the charge voltage rises the charge current is reduced. Similarly to the U curve above, a rise in temperature caused by a high current must be prevented through an increase in charging voltage.
Wa – curve
Charging that follows the W curve, although this time charging stops after a specific amount of time.
Constant current tables
Manufacturers' constant current tables display at which max. cut-off voltage the battery can supply a constant current per cell (or per block of cells) relative to time.
Cut-off voltage
Describes the voltage measured between a battery’s terminals after discharge. The value for each specific battery will be provided by the manufacturer. Falling below the cut-off voltage can cause the battery to be destroyed. The cut-off voltage also depends on the battery’s load capacity. For very small loads, it is wise to maintain higher cut-off voltages to avoid damaging the battery.
Cycle
A cycle is a regular, repeated process during battery charging and battery discharge.
Cycle-resistance
Cycle-resistance describes how often a battery can be charged and discharged before it reaches the end of its life.
D
Deep discharge
Describes the condition of the battery after it has been totally discharged at a low current and has dropped below the cut-off voltage. Deep-discharged AGM batteries must be recharged within 12 hours, or they will be irreparably damaged. AGM GEL batteries must be recharged within 5 – 7 days.
Diffusion
Diffusion describes the physical process that creates a total mixture of two or more materials.
Direct current
Direct Current describes electricity that does not vary in terms of power or direction over time (e.g. electricity from an electro-chemical energy source).
E
Electrode
Consisting of an active material and a conductor, this conductive mass is where electro-chemical reactions happen inside the cell.
Electrolyte
Electrolyte is a solid, liquid or dissolved chemical compound that dissociates into ions, and that is moved in single direction under the influence of an electric field.
Electrons
Electrons are negatively charged elementary particles.
Energy
Describes work that is measured in watt-hours, and which is defined by voltage and capacity. The more energy a battery stores and can supply, the more work can be achieved with it.
Environmental factors
Describes factors that can significantly reduce the battery's working life when adjustments are not made according to the manufacturer's guidelines. In general, these are: vibration, shocks, ambient temperature, humidity and height.
Equalisation charge
Equalisation charges are necessary in some open battery types in order to equalise decreasing charge capacity. Equalisation charges are required at relatively long intervals (6 – 9 months), depending on the battery type and manufacturer guidelines. The appropriate voltage and current levels can be taken from manufacturers’ data sheets.
ETN numbers
Describe code numbers for standard lead (starter) batteries.
The “European Type Number” is the (almost) universally adopted successor to DIN specifications (DIN = German Industrial Standard). The numbers in the code contain all important battery information (voltage, capacity, product category and cold cranking current). The ETN number is comprised of 3 groups of numbers with 3 numbers each. These encode the following information:
ETN 536 046 030;
- 1. Group 5 3 6 = group A:
- The first figure denotes voltage, with numbers 1 – 4 being reserved for 6-volt batteries
- the number 5 for 12-volt batteries under 100 Ah
- the number 6 for 12-volt batteries above 100 Ah
- the number 7 for 12-volt batteries above 200 Ah
- 8 for specialist starter batteries
- 9 for drive, lighting and solar batteries
- The second and third figures describe the capacity of batteries in Ah, so:
- 5 3 6 = 12-volt battery with a capacity of 36 Ah (below 100 Ah)
- 6 3 6 is a 12-volt battery with 136 Ah (over 100 Ah)
- 7 3 6 is a 12-volt battery at 236 Ah (over 200 Ah)
- the number 8 for specialist starter batteries
- the number 9 for drive, lighting and solar batteries
- The second group 0 4 6 = group B:
This group of figures encodes information about measurements and special features (for of the container base, type of container lid, base plate, etc.) - The third group 0 3 0 = group C:
These 3 figures describe the cold cranking current × 10. For example: 030 × 10 = 300 A, 115 = 1150 A.
- The first figure denotes voltage, with numbers 1 – 4 being reserved for 6-volt batteries
The previous DIN codes, e.g. 5 36 46, are constructed from the same information but lack cold cranking current specifications. However, in normal speech people would ask for a battery, 12 volt – 36 Ah, or battery, 12 volt – 88 Ah. Here, it simply must be established first that we are referring to standard batteries available as typical commercial stock.
Eurobat
EUROBAT is an association that promotes the interests of the European battery industry. With 34 members who make up more than 85% of the battery industry in Europe, EUROBAT works to develop new battery solutions and renewable energy storage methods. In addition, it can offer recommendations about the definition of technical battery terms such as working life.
Explosive (hydrogen) gas
Hydrogen gas is produced when the battery is overcharged. A mixture of hydrogen and oxygen forms via a chemical reaction in the electrolyte. This mixture is highly explosive.
F
Final charge voltage
When a battery is completely charged, the float voltage that offsets the battery's self-discharge is the only remaining energy flow. Charging voltage is set between 2.26 V/cell and 2.29 V/cell depending on the battery type.
Float voltage
This constant voltage is applied to stop the battery self-discharging. The charging voltage is set at 2.26 V/cell and 2.29 V/cell, depending on battery type.
Float charging
The voltage required to keep batteries in a fully charged state is called the float charge. Standard values at 20°C: lead batteries – 2.23 V – 2.27 V, 1% per cell; NiCd batteries – 1.40 V per cell. It is imperative to follow the manufacturers’ guidelines. If temperatures deviate from the norm where the battery is kept, either permanently or consistently, then the above values will change according to the manufacturer’s specifications.
G
Gases
When the battery is overcharged, it can lead to gassing – which means hydrogen coming out of the battery, and the corresponding risk of explosion.
Gassing voltage
The voltage at which the electrolyte in a battery is converted into a gaseous state and escapes. The voltage should not stay at the gassing voltage too long, as there would be a significant amount of electrolyte lost, and it could destroy the battery. Typically, the gassing voltage is around 2.4 V/cell in lead batteries and about 1.55 V/cell in nickel-cadmium batteries.
Gel battery
With its frequent discharge cycles and starting ability in all weather conditions, a gel battery has an ability to power a range of electrical devices. Motorbikes are one good example; driving on vibration-heavy dirt track, racing round corners, installing the battery in different positions, seasonal use with long periods of downtime and irregular charging are all instances where a battery using specialised GEL technology is required.
With the electrolyte bound by the gel, state-of-the-art GEL technology offers outstanding vibration-resistance. With the electrodes and liquid acid securely preserved in the multi-component gel, this set-up guarantees excellent cycle-resistance in a wide range of discharge situations, as well as the best leak-proofing. An internal gas recombination process prevents gas build-ups and allows the battery to be sealed. A pressure-regulated valve ensures that it meets the highest safety standards, even when overcharged.
GroE batteries
GroE batteries are closed, stationary lead batteries with liquid electrolyte (containing dilute sulphuric acid). Their construction is distinctive – they have completely casted plates with a lamellar structure. Their lead technology, high electrode strength and low acid density of 1.22 kg/l mean that they have an exceptional working lifespan of at least 20 years.
GroE batteries are manufactured with a range of capacities from 75 Ah to 2600 Ah. Their positive electrode is shaped like a large plate. The electrolyte is in liquid form and needs to be monitored whilst the battery is operating.
The number of cycles is over 200. The GroE battery range has the very best reliability, and they are extraordinarily safe to operate. The batteries also manage high voltage at a high discharge current, as well as a high level of consistency in their electrical properties throughout their working life.
The GroE has been used for more than 100 years and is therefore one of the most developed and secure types of battery system.
GRS
Stands for “Gemeinsames Rücknahmesystem” (the German National Battery-Collection Foundation).
The GRS foundation regulates the proper disposal of used batteries throughout Germany. Whether you're an industrial end-user or a public waste disposal carrier, GRS provides services that cover the pick-up, sorting and evaluation of old batteries.
H
High current charging
Charging where the current is at or above 1°C.
High current discharge
Discharging where the current is above 5°C.
I
Industrial battery
Rechargeable batteries that are exclusively for industrial, commercial and agricultural uses (e.g. for fork-lift trucks, hybrid vehicles).
Inner resistance
Ohmic resistance of a battery.
I/V Curve
The I/V curve is a tool to enable careful charging. First, the battery is charged at a constant current until the float (trickle) voltage is reached. After this, it is charged at a constant voltage.
L
Lead battery
A rechargeable battery that uses weak sulphuric acid as an electrolyte. The electrodes are made from lead.
Lead sulphate (PbSO4)
A chemical compound that forms at the plus and minus plates of a lead battery during discharge. It is formed by the chemical reaction between sulphuric acid and lead oxide at the positive electrode, and lead metal at the negative electrode.
Life (battery life)
Battery life depends on several factors, but the battery must be maintained according to user manual guidelines to achieve maximum longevity. The battery life is directly dependent on the following factors: the charging of the battery, the temperature of the surrounding environment, the number of charging cycles and deep discharges.
Lithium ions
The battery cell contains lithium ions in the positive electrode, the negative electrode and the electrolyte.
Low battery
A warning signal about an upcoming deep-discharge curve. It is used on some UPS installations as an advance warning at the interface or via the relay board.
M
Maintenance free batteries
A battery where the electrolyte is in gel form or in a micro glass-fiber mat (AGM). The battery is sealed and does not need to be filled with distilled water.
Memory effect
The memory effect describes a loss of capacity in the battery where it remembers the input-energy needed after a previous discharge, rather than the actual one. If the voltage of the cells falls below the minimum level, the battery will become unusable even though it still has electrical energy.
N
Nickel-cadmium
In a nickel-cadmium battery, one electrode is made of nickel and the other from cadmium. These rechargeable batteries are highly resistant to deep discharge and over-charging, and the cells are also cycle- and temperature resistant. NiCd batteries are banned for most uses, with battery regulations restricting their usage to bicycles, emergency lights, alarm systems and medical devices.
Nickel-metal hydride
Consisting of a positive electrode of nickel hydroxide and a negative electrode of metal hydride, NiMH batteries offer about twice the energy density as NiCd batteries at the same voltage.
Nominal capacity
The usable capacity of a battery under the conditions given by the manufacturer.
O
OGi battery
The OGi battery is a low maintenance, wet-cell battery with a liquid electrolyte. The design life of the battery is up to 15 years with an ambient temperature of 20 – 25°C. The maximum ambient temperature is -20°C to +50°C. These batteries are constructed with long-lasting positive grid plates, fine grain pasted negative grid plates, micro-porous separators and containers made from a stable, flame-retardant, crystal-clear SAN material.
The batteries are assembled as individual blocks consisting of several cells, or as single cells. Their rates of charge range from 25 Ah – 900 Ah depending on the model and size. The round mesh construction of the plate grid lowers the internal resistance of the battery, which means that the OGi can supply a very high current in a very short space of time. The cycle number of the individual charges/discharges is > 1000.
OPzS batteries
OPzS batteries are wet cell lead batteries with a liquid electrolyte (diluted sulphuric acid). The tubular-plated construction of OPzS batteries offers a very high cycle life at up to 1500 cycles at 80% depth of discharge. Hence tubular-plated batteries are ideal for applications where a high charge/discharge capacity is needed – e.g. in solar applications or where there are long bridging times, such as in IT/telecommunications and emergency lighting. Their typical working lifespan is around 20 years.
Applications:
- IT/Telecommunications
- Emergency lighting
- Solar and UPS
- Back-up batteries, BEV technology
- Wind installations
Advantages:
- High charge and discharge capacity
- High cycle resistance and longevity
- Exceptional reliability
- Very suitable for long bridging times
OPzV batteries
OPzV batteries are low maintenance, sealed individual cells that are usually made with plastic containers. The positive electrode is a tubular plate. Smaller capacity batteries up to ca. 300 Ah are available in 12 V models. The batteries’ working lifespan is typically over 15 years. The batteries are produced with capacities of up to 3200 Ah. The electrolyte is in gel form and does not need to be monitored during the battery’s life. They are best employed for uses where a bridging time of more than half an hour is necessary. As well as a long working life in parallel standby mode, they also have a cycle-resistance that is almost as high as in OPzS batteries.
Therefore tubular-plate OPzV batteries are ideal for usage in applications where a high charge/discharge capacity is needed, e.g. IT/telecommunications, emergency lighting, UPS, BEV and wind-power installations.
Advantages:
- High current capability
- Horizontal installation option
- High cycle-resistance and working life
- Minimal maintenance
- Excellent reliability
Overcharging
A battery is overcharged when the chosen charging voltage is too high. Initially, the lead sulphate is converted back to lead and lead oxide, but because the charging current continues to flow, the lead on the grid also starts to be affected. This causes the grid to expand and weakens the press-fitted materials.
P
Parallel connection
Several battery blocks or wires are connected with each other to increase capacity. See also: Parallel switched battery cables.
Parallel switched battery cables
When connecting several batteries in parallel, it is important to make sure that the cabling between individual battery wires is symmetrical. It’s best to make them all the same length, so that the charging voltage of individual wires is the same, and no battery receives a charging voltage that is too low or too high.
Polarity
This term describes the relative charge or voltage (opposing) between two electrodes.
Positive terminal
Positive connection on a cell or battery.
Primary cell
A non-rechargeable cell.
R
Residual capacity
The amount of capacity left in the battery after it has been discharged. The faster a battery is discharged, and the higher the discharge current, the higher the residual capacity in the battery will be.
Residual charge
A full charge from an undefined state of charge.
Round cell
A round cell, in contrast to a prismatic cell.
S
Sealed batteries, maintenance free
These are batteries with a gas recombination rate of at least 95%. I.e. it is not necessary to refill them with water throughout their entire working life. The batteries are generally described as “maintenance free”.
Secondary battery
A rechargeable battery.
Self-discharge
Describes the battery's own discharge that is independent of connection to an electrical device. Self-discharge is a constant process of chemical reactions that depends on the temperature.
Separation
A separator ensures that the positive and negative electrodes (cathode and anode) remain isolated from one another.
Serial connection
Connecting the negative terminal of a battery to the positive terminal of another battery. The combined pair has double the voltage at a constant capacity.
Short circuit current
This describes the current that flows through the terminal of the battery during a short circuit. It is only limited by the internal resistance of the battery. Short circuit currents should never be underestimated, and they can increase to several thousands of amps in large batteries. (For example: a battery at 65 Ah can supply a short circuit current of up to 1500 A). With short circuits there is always the possibility of burns being caused or objects being set on fire. A total short circuit at the battery terminals can cause the battery to explode, which is why every battery must be fitted with a suitable fuse.
Standby
In a standby set-up, the battery is usually connected to an electric appliance in parallel, and it is only discharged when the normal power source fails, and the emergency power system is initiated.
Sulfation
When a lead battery is in a state of deep discharge, a process of recrystallisation can lead to the formation of coarse-grained lead sulphate at the electrodes. This reduces reactivity at the electrode's surfaces and can lead to short circuits when the battery is jolted.
T
Terminal type
The terminal type describes how the battery can be connected.
Traction battery
The traction battery is a drive battery.
U
UPS installation
UPS = Uninterruptible Power Supply. These installations are switched on during disruptions to the electricity supply so that electricity continues to be supplied without any outage.
V
Vented batteries, low maintenance
The electrolyte liquid in the individual battery cells can be topped up through openings in the battery case. This battery type is also known as an “open” battery.
Volt
The unit for electrical voltage, shortened to “V“. The term was coined in 1897 after the Italian physicist and medic, Duke Alessandro Volta.
Voltage delay
This describes a drop in voltage in lead batteries at the beginning of a discharge. This effect is highly dependent on how high the required current is. High load capacity batteries should therefore also be used. HR (high rate) type batteries are particularly useful in this regard.
W
Wet-cell batteries
Conventional wet-cell consumer batteries have an energy supply that is just as reliable as that of AGM batteries. However, wet batteries do need maintenance, with regular checks of the battery liquid and top-ups with distilled water needed to guarantee battery longevity.
Working life (battery)
A duration defined for batteries that describes how much capacity a battery still has to fulfil its function despite loss of capacity due to storage and temperature-related effects.

History of the battery's origins

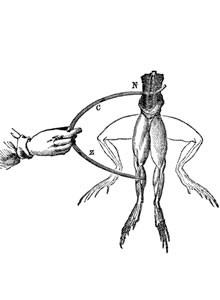

The beginning: The history of the electrochemical energy reservoir started with the scientific study of electricity
Luigi Galvani (1737-1798), who first discovered electrical phenomena, and Alessandro Conte di Volta (1745-1827), who developed the first voltage generator, are two names that are central to the story. They live on through terms such as the “galvanic cell” and “volt”. In experiments conducted in 1789, Luigi Galvani noticed that a frog’s leg began to twitch when it encountered two different metals. From this, he deduced a correlation between electricity and particular muscular functions.



Ten years later, in the year 1799, A.C. di Volta built the first basic battery: he assembled alternating layers of copper and zinc, stacked on top of one another, and between each layer he inserted a piece of cardboard soaked in a salt solution. When the layers were connected by wires, this “Voltaic Pile” created energy. And when several of the piles were connected in a row, the voltage increased.
In 1802, Johann Wilhelm Ritter, who was a scientific collaborator of Goethe’s, created a battery called the “Ritter pile”. The stack consisted of copper and cardboard layers soaked in a table salt (sodium chloride) solution. This apparatus could be charged with an electric current and produced electricity when discharged. The “Ritter Pile” is the forerunner of the modern battery.
Around 1840 to 1850, two men named W.J. Sinsteden and G. Planté began employing the first lead batteries (made of lead, sulphuric acid and lead dioxide), which were used to store energy for telegraph-related experiments. Both were using lead plates as electrodes that had been built up to a certain capacity through repeated charging and discharge. However, these batteries were not yet suitable for mass production.
Thanks to industrialisation, electrochemical energy storage began to develop quickly. Dynamos and bulbs were discovered towards the end of the 19th Century, and the demand for storing energy grew quickly. Around 1880, once C. Fauré had filed a patent for producing paste-covered plates for lead batteries, they started being manufactured on an industrial scale. Continuing in the same vein, W. Jungner and T. Edison followed in 1899 and 1901 with the nickel-cadmium battery, which could also be mass-manufactured very soon.
The lead battery’s early years
The physicist Fauré covered both sides of a lead mat with a paste made from lead powder and sulphuric acid. This enabled him to achieve a particularly high capacity after the first charge (the “formation”), which paved the way for the industrial production of the battery. In the wake of this, several companies were established. One of them was La Force et La Lumière S.A., where W. Thomson worked (who would later be known as Lord Kelvin of Largs, the man who gave his name to the absolute temperature scale). To begin with, the idea was to build giant energy stores, with W. Thomson conceiving a plan to supply electricity from the Niagara Falls to the town of Buffalo. With 80,000 volts (V) being generated and supplied to Buffalo via a 40,000-cell battery, households in the town would then be supplied with 100 V of network power with a tap of 50 cells each. Due to various reasons, however, the plan never came into fruition.
With their spiral-wound positive and negative electrodes, Fauré cells were not very durable and failed after only a few charge and discharge cycles.This was a major obstacle when it came to producing batteries on an industrial scale.
Electrode types

A major advance was made in 1881 – J. Scudamore Sellons had the idea not to apply the paste to a flat plate, but to work it into a perforated plate instead, creating better adhesion. He was the first metal scientist to use antimony alloy as a lattice material, something that would prove so important later. It’s not known to what extent each man knew about the other, but the same year, Ernest Volckmar developed a lead grid known as a "pasted grid" that soon became commercially available in many different versions.
That same year, Charles F. Brush registered a patent for a lead electrode with a large surface area and corrugated surface. Both these plate types (pasted lattice plate and large-surface plate) are still much in use today. Even the tubular plates now commonly used in Europe and Japan for traction and stationary batteries have a long history: S. C. Currie invented the basic form in 1881.
In the case of tubular plates, a lead rod about 8 mm in diameter lies at the center of the tubule – something that is still a key feature today. An outside layer of woven or unwoven material gives the active material mechanical assistance.
The special role of alloys in a lead battery
Because corrosion spreads gradually through the metal and turns lattice material into lead dioxide, this system is unstable, causing the grid to lose its mechanical strength and its conductivity. But a protective layer slows the corrosion to such an extent that if the battery components are well positioned, the battery’s operating time is not compromised.
The potential of the negative electrode lies at 0.35 V below the equilibrium potential of the hydrogen electrode. Under normal circumstances, hydrogen should be released from the weak sulphuric acid solution, discharging the battery. However, using lead significantly reduces the build-up of hydrogen, and the gas is produced very slowly. Thus “self-discharge” at the negative electrode cannot be stopped entirely, but it happens so slowly that it can be tolerated.
Pure-lead (refined lead) lattice alloys were derived from the large-surface plate dating back to Planté, with a small layer of active material being formed on a large cast via a process of electrochemical oxidation. Unalloyed lead is an unsuitable material for a light lattice because it is not solid enough for further work. Furthermore, pure-lead lattices or plates are almost impossible to manage in a standard assembly process.
In the USA, because of its geography and many relay stations spread over large distances, this posed a big problem – which was why BELL Laboratories was urgently looking for a way to overcome it. From 1935 onwards, research was carried out exploring the possibility of using lead-calcium alloys instead of lead-antimony alloys. Eventually, these were implemented in BELL’s fixed battery installations. Despite extensive pre-testing and field tests, they proved highly disappointing, with the operating life of the batteries frequently way below initial expectations.
To cope with growing lattice-sizes, BELL Laboratories created stationary batteries with saucer-like electrodes made from pure lead. In 1970, these were rolled out at BELL’s telephone installations.
The addition of metals contributing to the fine structure of the solidified material (fine-grained alloying) would prove important. Otherwise, alloys that contain small amounts of antimony can’t always be poured smoothly. Here, the addition of small quantities of selenium (200 g/ton) proved to be particularly effective; the selenium forms fine lead-selenide particles (PbSe) that act as foci during the solidification process, enabling crystal formation and allowing the desired finely grained structure to emerge.
Lead batteries made with these alloys have such low water loss that, for “stand-by” applications and under normal conditions, they will only need a refill after more than 5 years have passed. The remaining antimony content stabilizes the cycle sequence to such an extent that they can reach more than 1,000 charge/discharge cycles. Under normal operating conditions, standard batteries such as these – carrying the “maintenance free” DIN classification – need no extra water throughout their standard 5-year working life.
Further development of the lead battery
At the end of the 19th century the lead battery as we know it was already being manufactured including the three electrodes that are still the norm today. Development of the battery continued over the hundred years that followed, and better knowledge about determining factors permitted better production processes and new plastics to be used as material for separators and containers. We will only outline a few of these developments here.
Tubule electrodes work efficiently with the active material and offer a high level of cycle stability. In the early days, the tubules were made from slitted hard rubber, but after the second World War, braided glass fibers – a woven material made from glass fibre and other synthetic fibers, or a web or felt made from a pure plastic (polyester) – were introduced as a new material.
The valve-regulated lead-acid battery (VRLA)
Valve-regulated lead-acid batteries significantly reduce the need for maintenance. They work according to the same principle of the air-tight nickel-cadmium battery. The oxygen produced at the positive electrode does not leave the cell – instead, it is reduced back to oxygen ions (O2-) at the negative electrode and combined with hydrogen ions (H+) to form water. Thus, oxygen produced by overcharging the positive electrode is balanced by oxygen reduction at the negative electrode. If the internal oxygen cycle is running perfectly, there is no loss of water.
Since a certain amount of hydrogen build-up in lead batteries is inevitable, it is impossible to achieve perfect internal oxygen circulation – even at a cell’s electrically-neutral equilibrium potential. Another problem is the inevitable lattice corrosion at the positive electrode. Both secondary reactions limit how efficiently oxygen can circulate internally, making a certain amount water loss inevitable. For sealed lead batteries, this constitutes their principle point of difference compared to nickel-cadmium batteries.
To achieve an effective internal charging/discharge cycle, oxygen must reach the negative electrode as a gas. However, in a liquid electrolyte, the process would be too slow. Thus, it is achieved either by adding silicon dioxide (SiO2) gel – shrinking it and forming gaps through which the gas can pass – or the acid is absorbed by a glass mat of extremely fine glass fibers (with a micrometer(µm)-thin diameter). In these absorbent glass mats, gas can pass through large pores left unfilled by the electrolyte.
Attempts to make static electrolytes out of gel had begun even before the end of the 19th century. Like “dry batteries”, the idea was to prevent any spillage of sulphuric acid even if the container was breached. At the time, nobody had given much thought to applying this method to lead batteries, but in 1950 a battery company called “Sonnenschein” revived the idea, though at first the ambition was to make smaller tilt-proof batteries (which were equipped with valves and therefore shared some of the characteristics of valve-regulated lead-acid batteries).
In the seventies, a glass fiber mat with a µm-thin diameter began to be used, allowing valve-operated lead-acid batteries to be used more widely. Originally designed as a micro-filter, the ability of the material to absorb the sulphuric acid electrolyte meant that it could be used as a separator, preventing short cycles (short circuiting) between the electrodes and simultaneously holding the electrolyte in place. Another advantage of the technology was that the batteries could be assembled at conventional plants. Moreover, they have such low internal resistance that the batteries could supply high discharge currents very effectively. At the end of the seventies, valve-regulated starter batteries began to be used for cars. But for various reasons they were not a success, and the battery’s advantages as a starter were overlooked.
However, the battery proved very successful for telephone installations, and a trend was started of using sealed lead batteries for many stationary applications (for example, today most uninterrupted power supply installations are equipped with valve-regulated lead-acid batteries). This success was due not just to their low maintenance requirements and low quantities of hydrogen production, but also because they could safely be placed next to other electronic components without the danger of corrosive vapors leaking out of them.
Valve-regulated lead batteries continued to be developed with a jellied electrolyte (gel), and in the seventies the same principle was applied to larger batteries. Today, there are gel electrolyte batteries with a capacity of up to 3,000 Ah per cell, with various models for stationary and/or mobile applications. VRLA batteries have also been a step forward in environmental terms, because the electrolyte is kept locked in a glass mat, or in a silicate gel.
General development
Besides the specific developments above, there have been several significant more general lead-battery innovations over the decades.
First, spacers made of hard rubber or thin blocks of wood were used to separate the electrodes. In 1915, a porous separator made from hard rubber was patented. In 1924, a similar one was invented in Germany with latex as the base material. In both cases, the objective was to create a precise system of pores using elastic material and filler. With a few modifications, these are still in use today. After 1945, plastic was the main material used for separators – especially PVC and polyethylene. Likewise, battery containers were increasingly being made from plastics, instead of glass or hard rubber.
The constant improvement of electronic components has paved the way for better charging technology. Car batteries’ average state of charge could now be increased and battery life extended. Monitoring of stationary batteries has improved, preventing unexpected outages. In the last couple of years this trend has continued, and today you can get devices that continuously monitor the battery.




